Abstract
Introduction
Large cell transformation (LCT) in mycosis fungoides is defined as 25% large cells and is traditionally associated with a poor prognosis and a median survival of 3-5 years. There is no consensus on the appropriate management for these patients. We aimed to characterize clinical and pathologic features of patients with transformed mycosis fungoides (tMF) at our institution and to describe treatment patterns and patient outcomes.
Methods
We performed a retrospective review of 63 patients with a histopathologic diagnosis of tMF seen at the Winship Cancer Institute of Emory University from 1990-2021. Clinical data collected from the electronic medical record included demographics, baseline laboratory values, disease characteristics, pathologic characteristics, and therapy. Kaplan-Meier curves for OS and PFS were generated for the whole cohort. Descriptive analysis was performed for covariates. The association of baseline variables with OS was modeled by Cox proportional hazards model.
Results
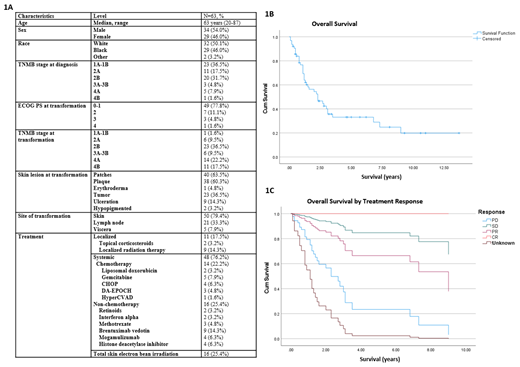
Among 63 patients with tMF, there was even distribution among male (54%) and female (46%) patients (Figure 1A). The median age at the time of large cell transformation (LCT) was 63 years (range, 20-87). This population included 46% African American (AA), 50.8% White, 1.6% other races (n=1). At the time of diagnosis, the stage was 1A-1B in 23 (36.5%), 2A in 11 (17.5%), 2B in 20 (31.7%), 3A-3B in 3 (4.8%), 4A in 5 (7.9%) and 4B in 1. The ECOG performance status (PS) was 0-1 in 49 (77.8%), 2 in 7 patients (11.1%), 3 in 3 patients, and 4 in one patient. Most patients had patches and plaques at diagnosis, 23 (36.5%) had tumors, and 9 (14.3%) had ulceration. Only 2 patients had hypopigmented MF. The most common therapies prior to LCT were topical steroids (n=34), phototherapy (n=25), topical nitrogen mustard (n=20), oral retinoids (n=14), and oral methotrexate (n=8). Radiation (RT) was received in 22 patients prior to transformation with 16 having localized RT and 9 total skin electron beam therapy (TSEB).
The median time from diagnosis to LCT was 2.1 years (range, 0-32 years). Most patients had advanced stage at the time of LCT (n=45, 86%), with stage 2B the most frequent 23 (36.5%). Other stages at LCT were 1A in 1 patient, 2A in 6, 3A-B in 6, 4A in 14 and 4B in 11. Overall, 47 (74.6%) patients experienced progression in their TNMB stage following LCT. LCT occurred in the skin in 51 patients (81%), lymph nodes in 21 (33.3%), and viscera in 5 (7.9%). The percentage of large cells was >50% in 24 patients (38.1%), and < 50% in 13 (20.6%). CD30 percentage was missing in the majority of patients (n=37, 58.7%). T-cell rearrangement in blood, skin, and lymph nodes was not consistently characterized with data missing in nearly half the patients (n=30, 47.6%). In those with TCR checked in at least one location, it was non-clonal in 19, had clonal persistence in 6 (9.5%), clonality in at least one sample but either not rechecked, or not persistent in 6 (9.5%), and oligoclonal in 2 (n=3.2). The most common treated for LCT was total skin electron beam therapy (TSEB, n=16) and chemotherapy (n=14), followed by brentuximab vedotin and localized RT in 9 patients each. Other treatments included topical corticosteroids (N=2), histone deacetylase inhibitors (n=4), and mogamulizumab (n=4). The median overall survival was 2.3 years from LCT (95% CI 1.1-3.5 years), and the median PFS was 2.6 years (95% CI, 1.1-4.1 years) (Figure 1B) . The median time to treatment following LCT was 30 days (range, -1 day to 2.3 years). By cox regression, neither baseline demographic factors, tumor characteristics, treatment, nor time to LCT were associated with progression-free or overall survival. Only response to treatment for LCT (complete response, partial response, or stable disease) was associated with survival (p=0.041, Figure 1C).
Conclusions
We characterized the clinical features and outcomes of a cohort of patients with tMF seen at Emory University over a 30 year period. We found poor outcomes despite many patients receiving novel and targeted therapies in the modern era. Key pathologic and clinical variables such as CD30 percentage and t-cell gene rearrangement studies were not reported for many patients, suggesting standardized practices are needed in the diagnosis and pathologic evaluation of patients with tMF. Additional pathologic correlatives will be updated at the time of the presentation.
Allen: ADC Therapeutics: Consultancy; Kyowa Kirin: Consultancy; Secure Bio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal